Sangamo's Technology Can Possibly Advance New Treatments For Patients With HIV
There is a small-cap biotechnology stock known as Sangamo Biosciences (SGMO) which has traded to a 52 week high of $24.69 per share and has since pulled back to $14.20 per share. We believe that this pull back presents an opportunity for long term investors to dip their toes into the stock. Sangamo is a biotechnology company focused on targeting unmet medical needs using engineered DNA-binding proteins.
ZFP Transcription Factors
Sangamo has developed two platform technologies with which it can fight against a variety of diseases. The first platform technology is known as ZFP Transcription Factors --ZFP TFs. These transcription factors are not natural occurring processes instead they are engineered by Sangamo to be equivalent to the natural body's gene processing mechanism or DNA. This is achieved by attaching a particular domain -- gene activation sequence or gene repression sequence -- together with a ZFP to generate a ZFP TFs. In essence this ZFP TFs which is engineered by the company can turn genes on and off the way it is created to. The functional goal for this technology is to be able to achieve two very distinct functions. The first function is to turn more genes back on. As an example when a certain neurological diseases destroys particular genes in the body, Sangamo's ZFP TFs would be responsible for reversing the destructive process of these genes and activating the genes back on thus stopping the disease. The second function would be to be able to turn genes off. Any disease that is overexpressed with a particular gene -- that is genes that are overexpressed in the patient's body that should not be there -- can be turned off by the ZFP TFs to eliminate the disease.
ZFP Nucleases
The second type of technology that Sangamo has produced is a technology known as ZFNs -- Zinc Fingered Nuclase. ZFNS are in essence ZFPs that are attached to an endonuclease that are cut in half. This endonuclease that is cut in half by the ZFPs forms the newly ZFN molecule. An example of a ZFN can be seen in the graphic below:
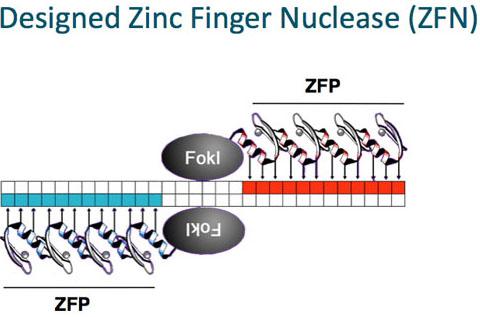
Source: www.sangamo.com/technology/zf-nucleases.html
As you can see in the image above the ZFN is cut by two ZFP molecules. This cut in the middle by the ZFP molecules is known as cleavage. This cleavage occurs so that the company is able to achieve a couple of functional goals for their technology. This cleavage is performed so Sangamo's technology can achieve its proper function. This proper function involves the cleavage of the ZFN molecule so that it can attach to a new DNA cellular structure. The company made sure of this by enacting the ZFP to be able to attach to its intended gene target dependent on what disease the company is targeting. The usefulness of being able to cleave the DNA can be advantageous for the fact that the company has three options that can be achieved upon cleavage of said ZFN molecules. These three options are shown in the graphic below:
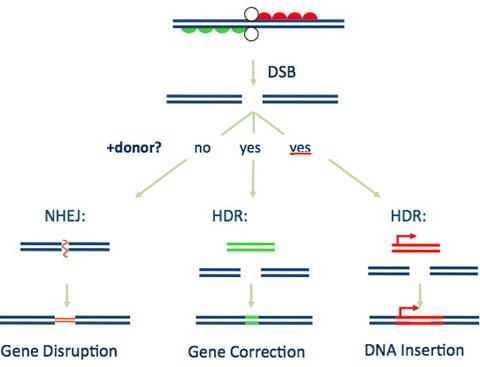
source: www.sangamo.com/technology/zf-nucleases.html
Gene Disruption
The first thing that can occur is in the graphic above titled under "Gene Disruption". This occurs when two DNA strands are broken apart which in turn allows DNA genetic material to be lost, but the DNA molecule is able to repair itself back together. With gene disruption Sangamo can engineer a gene that can interfere with the genetic code of the targeted disease. For instance Sangamo is working on developing a compound for HIV that uses CCR5 genetic code to function. The company could slip in a genetic code similar to the CCR5 gene that could disrupt the current flow of the disease.
Gene Correction
The second example deals with Gene Correction as shown in the graphic above. Under Gene Correction the DNA genetic material is lost as shown above under the Gene disruption between the two ZFP molecules, but Sangamo can incorporate a coded DNA. This coded DNA is known as a "Donor" DNA that is able to copy the old genetic material lost in the process of the cleavage and then incorporate it back to the ZFN -- remember ZFN is the two ZFP molecules cleaved together. The underlying method for Gene correction is for the ability to target diseases that require repairs in genetic mutations. By utilizing Gene Correction the company can alter the genetic material slightly but keep it similar to the targeted disease. Such diseases that could be targeted with this method could include: Hemophilia and Sickle Cell Anemia.
DNA Insertion
The final example that can occur which is also shown in the graphic above is DNA Insertion. In this step the company has to do a little engineering work. By that we mean they have to engineer a gene-size segment of DNA and then encode it to a particular location. As you can see in the graphic above under "DNA insertion" the gene segment has to be added a particular location. But with this method the company can incorporate their own DNA molecule they would like to add that can alter the DNA of the targeted disease.
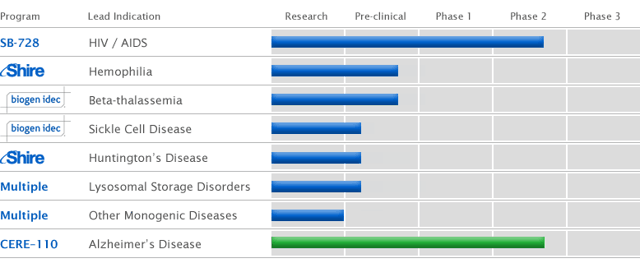
A Pipeline With Multiple Technologies
With all these technologies Sangamo has many shots on goal and many different types of diseases that it can target. Just think about how many diseases can be treated by altering genetic code. A change in the genetic DNA code would be able to possibly eliminate diseases from advancing into advanced stages, and maybe the possibility to cure them altogether.
(click to enlarge)
source: www.sangamo.com/pipeline/index.html
The company is targeting many unmet medical needs with their product platform. Which as described above can be used with great flexibility because of the different methods in which these diseases can be altered. This article will focus on the HIV/AIDS program known as SB-728-T which is currently in early stage clinical testing.
HIV/AIDS
HIV is a virus that infects a person's immune system and renders it useless against fighting off other infections and diseases. Viruses can normally be cleared by the body, but not when the body is infected with HIV. For instance the Flu virus targets your immune system but eventually the immune system is able to overtake the flu virus. The problem with HIV is that the virus is able to take over the T-cells -- immune system -- and then produce more copies of itself before it destroys other T-cells. Scientists aren't quite clear on why the immune system is able to clear most other viruses but has a hard time clearing the HIV virus. There are more than 1.1 million people in the United states that are living with HIV. There are also 1 out of 6 people in the U.S. that are not aware that they even have the HIV infection in their bodies. This is because the HIV takes time to invade the immune system over the course of many years. Eventually the immune system starts to break down and lose out to the HIV. That is the HIV starts to break down the CD4 cells which are responsible for defending the body against other infections. This break down of your immune system causes your body to not be able to fend off against other diseases. This reduced immunity to infections eventually leads to the AIDS Virus.
As mentioned above the HIV infection can lead to AIDS over time, but has to go through two other stages first. These two stages are: Acute HIV Infection and Chronic HIV Infection. Acute HIV Infection is where the Virus starts to attack the CD4 immune cells of the body and can take up to 3 months to develop into this stage. This stage produces minor symptoms like Flu, Fevers, and Headaches. The next stage which is Chronic HIV Infection is where the virus continues to multiply itself in the body and continues to destroy the patient's immune system. At this Chronic stage though the patient has not lost full functionality of the immune system. This stage of the virus can last up to 10 years which is very long time.
The possibility here is for Sangamo to produce a DNA treatment that would be able to prevent the Virus from continuing to copy itself and destroy more immune system CD4 cells. The prevention of the Virus advancing would block the ability for the patient to develop their HIV into AIDS which in turn could save many lives. For now if the development of the Chronic HIV Infection is not halted the Virus progresses to AIDS which at the point the patient's immune system has pretty much been destroyed completely. This prevents the immune system from fighting even the most minor infections which can lead to death. There are about 1.15 million people in the United States that have the AIDS virus.
SB-728-T
SB-728-T is being developed for the treatment of patients with HIV. What makes it remarkable though is the possibility of being given the possibility of being a "functional cure for HIV". As mentioned above in the "Gene Disruption" sub section Sangamo is looking to slip a new gene that will be repaired with the new DNA. The company wants to do this because it wants to mimic the response that currently occurs in patients who are resistant to the HIV virus. These patients are resistant to the HIV virus because they have a known "CCR5Delta32" gene which is a non-functional CCR5 protein. Those infected with HIV are susceptible to the virus, because their cell's surfaces are encoded with a functional CCR5 protein. By Sangamo utilizing the gene disruption they can break the mode of the CCR5 gene, and cause the gene to repair with the CCR5Delta32 gene back inside. The function here would be to make all patients resistant to the HIV disease thus creating a "functional cure" of the disease.
On March 6th 2014 Sangamo shares soared as much as 23% in one day after promising results were released at the CROI 2014 Conference. The results of the study showed that SB-728-T continued to show control of the HIV viral load for 31 weeks straight. These results are promising, because this sustained viral load in patients with HIV was done without using any ART -- antiretroviral therapies. Sangamo also employed a different strategy and has found a way to increase their SB-728-T therapy by using another single agent therapy first. Sangamo decided to first infuse patients with Cytoxan -- a single agent chemotherapy compound from Bristol-Myers Squibb (BMY) -- which first clears the patients' T-cells. The company noticed increased efficacy results by first using Cytoxan to clear patients' T-cells and then follow up with SB-728-T thereafter. These positive results can be described in this quote by Sangamo's Executive Vice President:
"Using our ZFN genome editing technology we can engineer immune system cells to achieve control of the virus without ART"
The quote above describes the power of the ZFN technology that is able to allow patients to control their HIV viral load in their bodies without the need to use any type of antiretroviral therapy. These results are promising and the company expects to add an additional 6 patients to this study. The reasoning for this is to find the highest Cytoxan dose possible without affecting patients with severe side effects. We think that this can further increase the efficacy of the compound so investors should pay close attention to these results when they are released.
Market Opportunity
There are a significant amount of people infected with HIV and AIDS worldwide as we have mentioned above. If Sangamo gets its compound approved by the FDA it will be able to obtain a large portion of the HIV market. Of course it will also have to produce efficacious results that are greater than the current therapies out in the market. Gilead Sciences (GILD) has a couple of products on the market that are meant to treat patients with HIV. The three drug compounds are: Atripla, Truvada, and Stribild. Gilead has obtained some great Q4 2013sales from their new HIV drug Strbild, and sustained sales on older HIV drugs. Atripla sales had rose by 2% that quarter to $933.6 million dollars. The middle stage HIV drug has seen a minor loss of 2% to $832.7 million for the quarter but despite the small dip that it still a good chunk of sales for the HIV market. The newest drug from Gilead to treat patients with HIV is Stribild which obtained sales of $203.8 million dollars which is a whopping 409 percent increase that quarter.
Sangamo may have some heavy competition from Gilead, but that remains to be seen depending upon the remaining trials. If Sangamo establishes greater efficacy in their primary endpoints of the trial then there shouldn't be a problem stealing market share away from Gilead. On the flip side if Sangamo produces similar or less than stellar results it could have a hard time taking any kind of Gilead's market share. As with all biotech stocks that is the risk of investing in a small-cap biotechnology stock that has no established drug.
Moving forward investors should keep an eye on new compounds from other pharmaceutical companies that are also attempting to enter the HIV/AIDS drug market. As of November 2011 the HIV/AIDS market reached $13.5 billion dollars in the seven top markets. These seven top markets are: United States, United Kingdom, Germany, France, Italy, Spain and Japan. The expected rate of growth is around $1 billion dollars per year so as of 2014 the HIV/AIDs market now totals (3 years X 1 billion per year) = 3 billion dollars. Taking the 2011 number of $13.5 billion and tacking on the $3 billion dollars will net $16.5 billion dollars for the HIV/AIDS market size as of 2014.
Financials
According to the 10-k SEC filing Sangamo Biosciences has $131.8 million dollars as of Feb 24, 2014. The company expects to be in a comfortable position to run through the rest of 2014 without having to raise any additional cash. This in part has to do for the fact that the company recently raised money back on March 20, 2014 obtaining approximately $100 million dollars to fund clinical operations, and for other related expenses. Investors should keep in mind though that this is not the only reason why Sangamo is in a comfortable cash position with a significant cushion. The other reason has to do with two significant partnerships with other pharmaceutical companies that have shown great interest in Sangamo's pipeline.
The first company to express interest was Shire PLC (SHPG) which has given Sangamo an upfront milestone payment of $13 million dollars for their research collaboration. There is also the possibility for Sangamo to even receive additional financing if other milestones are met over the course of the collaboration agreement. Best of all Sangamo might also be eligible to receive royalty sales for any FDA approved products. These royalty sales on any FDA approved products will be given as double-digit royalty payments which should bring in Sangamo considerable revenue. This partnership was established because of Shire's interest in Sangmo's gene-editing technology described above.
Another deal which was later executed in 2014 was Sangamo forming a collaboration agreement with Biogen Idec (BIIB) to produce treatments for Hemoglobinopathies. These Hemoglobinopathies are inherited conditions in which patients are unable to produce enough Hemoglobins in the body. Without these Hemoglobins patients are unable to carry enough oxygen through the blood stream. Sangamo has received an upfront milestone payment of $20 million dollars for this agreement. Biogen is also obligated to reimburse Sangamo for all related costs for the trials that are to be performed. Sangamo may be eligible to receive up to $300 million dollars upon certain milestones being met. These include: Development, regulatory, commercialization, and sales milestones. We believe this will be a huge surge in funding if these milestone achievements can be met on a continuous basis.
Risks
As with all biotech stocks there are many risks associated with investing in this sector, these risks are:
- Sangamo has not completed phase 2 testing, so there is no guarantee that the phase 2 results will come out positive
- Even upon positive phase 2 results the company will still have to complete a regulatory phase 3 trial which may or may not disseminate positive results for HIV patients
- Positive results from the clinical trials may or may not produce greater efficacy than current therapies being sold in the market
- With Positive results the compound's approval will be decided upon by the FDA. The FDA may or may not agree with the safety and efficacy produced from the final phase 3 HIV results
- Biotech stocks have seen a heavy sell off in recent weeks so there is a chance the stock may decline further than its current share price
- Sangamo may lose revenue if the milestones of the Shire PLC collaboration agreement are not met
- Sangamo may lose revenue if the milestones are not met from the Biogen collaboration agreement
Conclusion
We believe that Sangamo provides investors with the opportunity to enter Sangamo for the long term, and invest into the possibility that the company will come up with a "functional cure" of HIV. Results to date show continuous control of HIV viral loads in patients without the use of antiretroviral drugs. The company is well established with a great pipeline of drug compounds, and has already established partnerships with big names in the industry. We think that further results of the HIV compound will act as excellent catalysts to push the share price higher in the long term. We think that Sangamo is a great buy and hold in one's biotech portfolio.
I have no position in the stocks mentioned